Integrated management system in use: Clean work - also in quality management
Skan AG from Allschwil in Switzerland, one of the leading suppliers in the field of pharmaceutical isolators, is one of the pioneering companies in the specialist areas of cleanroom equipment and construction of cleanroom isolators for the pharmaceutical industry. Skan AG products are designed for aseptic or aseptic-toxic applications and meet the highest cleanliness requirements. The equipment [...]

Implementation of the guidelines for "good working practice" simplified
The choice finally fell on the ConSense GxP Enterprise software solution. This is tailored to companies from strictly regulated industries, including medicine, medical technology, pharmaceuticals and healthcare. As an integrated management system, the software facilitates the implementation and fulfillment of the guidelines for "Good Working Practice" and supports compliance with various national and international guidelines, laws and standards with many helpful functionalities. These include the use of electronic approval workflows, complete documentation, reliable access protection through a detailed role and rights concept, the option of integrating online tests and training, and the creation of capability profiles that provide information on the status of employee capabilities. The enterprise version of the software is also tailored to organizations with multiple locations and more complex structures.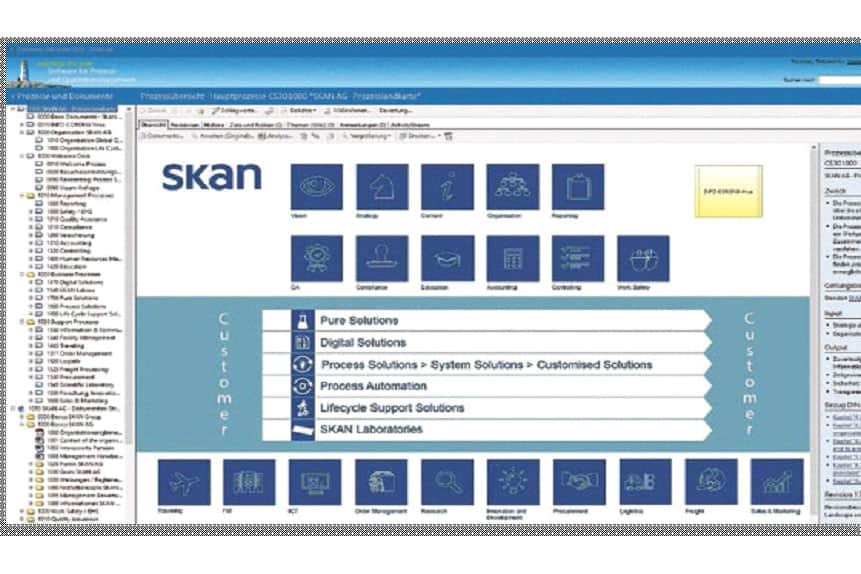
With software-based system to paperless documentation
The integrated management system now makes everyday work at Skan easier. It automatically compiles the relevant information for each of the more than 530 employees at the Allschwil site. The role and rights system stored in the system also regulates the distribution of content on an individual basis. Employees only receive the information they actually need in the current and valid version. Changes are communicated via the system, which prompts users to confirm that they have taken note of them. Searching for information is now also easier and faster for Skan employees, as Christian Flüeler recounts: "The ConSense software offers different ways here: Either you use the search function or you simply click on the corresponding sub-process in the process map. Links then take you to the stored information and documents. This is quick and easy and has contributed to a significant increase in employee acceptance of our QM system," says Christian Flüeler.Modular system, adapted to needs
The ConSense software is modular in design. Users like Skan can add many different modules to their system - e.g. audit management, measures management, training management and many more - so that it covers their individual requirements. Among other things, Skan uses ConSense measures management, which is used to centrally record and manage all measures arising in the company, for example, from audits, complaints, improvements or numerous other sources. "The software now makes it very easy for us to monitor fixes. While Skan has always been good at fixing bugs quickly, everyone knows this: sometimes details are still missing, which then get left behind due to workload. This is unacceptable in the pharmaceutical industry because everything must be documented correctly and completely. In the past, this was extremely tedious and took place with the help of Excel spreadsheets and paper forms. Now it all happens electronically. The system does not tolerate the unfinished measures, reminds the responsible persons and issues reminders. This helps us a lot to complete measures cleanly - especially from error reports - to document them completely and to check their effectiveness," says the quality manager. Another module, ConSense training management, was one of the reasons why the manufacturer of insulators decided to use the software from Aachen, as Christian Flüeler explains: "Our products are used, among other things, for the production of vaccines. Maximum safety is a must here. For this, we have to prove that our employees in production know the processes and documents exactly. In our Skan Academy, regular classroom training courses are held, which must then be documented so that this proof can be provided at any time. We now also regulate this via our integrated management system." The module provides an optimum overview and promotes efficient processes and a structured organization. In the case of recurring training measures, the software automatically reminds users of the necessary refresher courses. Christian Flüeler is glad that he now has an electronic management system that offers the flexibility to adapt such changes quickly and with little effort: "Overall, our structures have become much more complex. Today, we wouldn't even have the possibility to master all processes without a software-based system."Author
Dr. Iris Bruns is Managing Director of ConSense GmbH, Aachen (Germany). Another case study from her can be found here. More information: www.consense-gmbh.deThis post originally appeared on m-q.ch - https://www.m-q.ch/de/integriertes-managementsystem-im-einsatz-saubere-arbeit-auch-im-qualitaetsmanagement/